FOOD - DRUG INTERPHASE PRODUCTS
This guide serves to assist in determining if a product is to be regulated by the National Pharmaceutical Regulatory Division (NPRA) or by the Food Safety and Quality Division (FSQD) of the Ministry of Health Malaysia.
Introduction
It is important to monitor and regulate the marketing and sale of these products so as to protect the interest and health of the consumer. Some of these products are not clearly defined as “food” or “drugs” but are freely marketed. Such products include a variety of so-called health products and have been termed as “food-drug interphase (FDI) products”.
In order to better define and regulate the FDI products, both the NPRA and the FSQD, Ministry of Health Malaysia formed the Committee for the Classification of Food-Drug Interphase Products in 2000. The main Terms of Reference of the Committee is to assist both Divisions in classifying, in a consistent manner, an application from the industry which is not clearly defined either as a food or drug product. The Committee also serves as a platform in strengthening and updating the relevant regulations as well as to provide scientific input on these products.
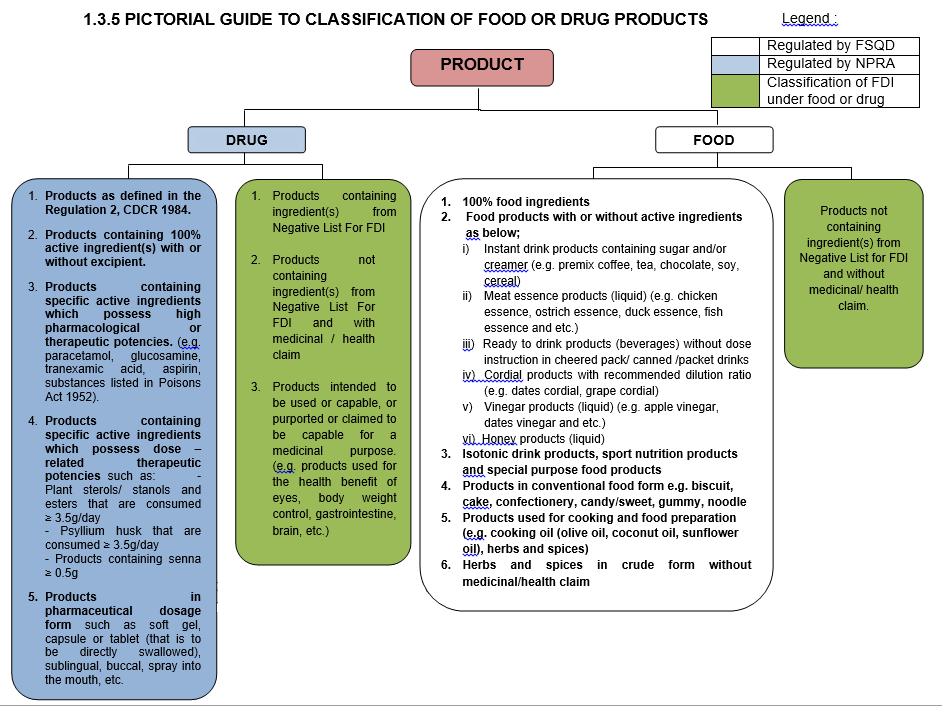
FOOD PRODUCTS THAT ARE NOT CATEGORIZED AS FDI PRODUCTS AND REGULATED BY FSQD INCLUDE :
1. 100% food ingredients.PRODUCTS THAT ARE NOT CATEGORIZED AS FDI PRODUCTS AND REGULATED BY NPRA INCLUDE :
1. Products containing active ingredient(s) with or without excipient ; orIt is important to determinethe category of a product
Classification criteria as below :
- FDI products containing ingredient(s) from Negative List for FDI shall be regulated by NPRA ;
NEGATIVE LIST FOR FDI
Table 1: Negative List For FDI
| No. | Ingredient | Common/Other name |
| 1 | Actaea racemosa | Black Cohosh, Cimicifuga racemosa |
| 2 | Antiaris toxicaria (Pers.) Lesch. | Bark cloth tree, antiaris, false iroko, false mvule, upas tree |
| 3 | Artemisia Spp. (all species) | Wormwood, Mugwort |
| 4 | Aspidosperma Quebracho-Blanco Schltdl | Kebrako, White Quebracho |
| 5 | Atropa Spp. (all species) | Antropa belladonna (deadly nightshade) |
| 6 | Azadirachta indica | Nimba, Neem |
| 7 | Bile | |
| 8 | Brucea javanica, Brucea amarissima | Sumatrana amarissimus, Java brucea |
| 9 | Bufo gargarizans Cantor, Bufo melanostictus Schneider, Bufo vulgaris Lour | Toad, Samsu, kodok, kerok |
| 10 | Calotropis Spp. (all species) | Apple of Sodom, Crown flower |
| 11 | Cannabis Spp. (all species) | Marijuana, Hemp |
| 12 | Catharanthus Spp. (all species) | Periwinkle |
| 13 | Chelidonium majus | Celandine, Great Celandine, Nipplewort |
| 14 | Chondodendron Spp. (all species) | |
| 15 | Claviceps Spp. (all species) | Ergot |
| 16 | Colchicum Spp. (all species) | Autumn crocus, Meadow saffron, Naked lady |
| 17 | Conium maculatum | Hemlock |
| 18 | Coptis chinensis, Coptis teeta | Chinese Goldthread |
| 19 | Croton tiglium L. | Croton |
| 20 | Datura spp. (all species) | Jimson weed, Devil’s apple, Green Dragon, Zombie’s Cucumber, Moon Weed, Trumpet Lily, Stinkweed |
| 21 | Digitalis spp.(all species) | |
| 22 | Dioscorea Hispida | |
| 23 | Dryobalanops lanceolata Burck | Borneo camphor, Kapur, Malay Camphor, Sumatra camphor |
| 24 | Dryopteris Spp. (all species) | Mountain woodfern, Spinulose woodfern, Spreading woodfern, Fancy fern |
| 25 | Euphorbia Spp. (all species) | Spurge |
| 26 | Fritillaria spp. | Fritillary Bulb |
| 27 | Gamma-amino Butyric Acid (GABA) | |
| 28 | Garcinia Morella Desr. | Gamboge |
| 29 | Gelsemium semperi virens | Palaung Thay |
| 30 | Glucosamine | |
| 31 | Glutathione | |
| 32 | Gypsum Fibrosum | |
| 33 | Hyaluronic acid | |
| 34 | Hyoscyamus Spp. (all species) | |
| 35 | Hypericum perforatum | St. John’s Wort |
| 36 | Juniperus sabina | Savin, Savine |
| 37 | Mahonia aquifolium, Mahonia repens, Mahonia nervosa | Mahonia Aquifolium: Oregon Grape, Mountain Grape, Barberry. Mahonia Repens: Creeping Barberry, Creeping Mahonia, Creeping Oregon-Grape |
| 38 | Melanorrhoea usitata Wall. | Vanish tree |
| 39 | Monascus purpureus | Red yeast rice |
| 40 | Mucuna pruriens | Cowhage, Cowage |
| 41 | Mylabris phalerata, Mylabris cichorii | Blister beatle, Mylabris |
| 42 | Natto extract | Fermented soy bean extract |
| 43 | Nerium indicum | Indian oleander, Exile Tree. |
| 44 | Nerium oleander | Indian oleander, Exile Tree. |
| 45 | Pearl | |
| 46 | Phellodendron amurense, Phellodendron chinense | Amur Cork tree |
| 47 | Placenta | |
| 48 | Plumbago indica | Rose-coloured leadwort |
| 49 | Plumbago zeylanica | White leadwort |
| 50 | Psilocybe cubensis | Boomers, Gold caps |
| 51 | Rauvolfia Spp. (all species) | |
| 52 | Resveratrol | |
| 53 | Sanguinaria canadensis | Bloodroot, Indian Paint |
| 54 | Scilla sinensis | |
| 55 | Simmondsia Chinesis | Jojoba |
| 56 | Sophora tomentosa | Sea coast Laburnum, Silver Bush |
| 57 | Spigelia marilandica | Worm grass, Pinkroot |
| 58 | Stichopus spp. | Gamat |
| 59 | Strophanthus spp.(all species) | Kombe |
| 60 | Strychnos ignatii, Strychnos lucida, Strychnos roberans | Nux-vomica |
| 61 | Symphytum peregrinum | Comfrey |
Notes:
This list :
- is a compilation by the FDI committee.
- is not meant to be exhaustive and will be reviewed from time to time.
- shall be read in conjunction with the current laws and regulations together with other relevant legislations, where applicable, governing pharmaceutical and natural products for human use in Malaysia
- FDI products not containing ingredient(s) from Negative List For FDI and with medicinal/ health claim shall be regulated by NPRA ; or
- FDI products not containing ingredient(s) from Negative List For FDI and without medicinal/ health claim shall be regulated by FSQD.
-
When there is greater uncertainty regarding the safety of a FDI product, such shall be regulated by NPRA. This is to enable closer monitoring of such products, so as to safeguard the health of the consumer.
Reference : Pekeliling Kriteria Baru Pengkelasan Produk (07 August 2014)
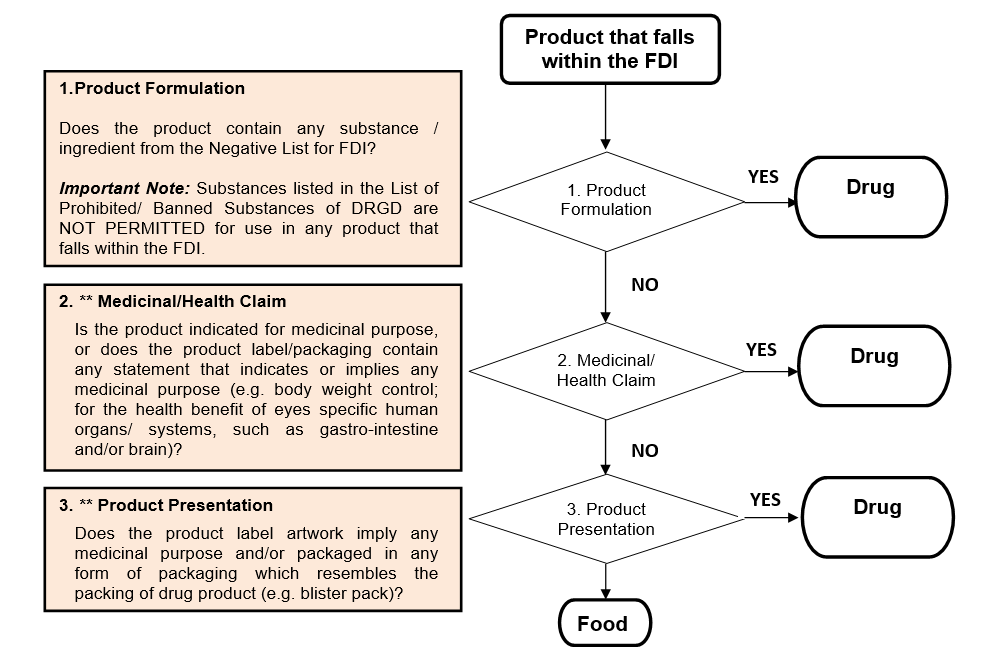
GENERAL CLASSIFICATION FLOWCHART OF FOOD-DRUG INTERPHASE (FDI) UNDER FOOD OR DRUG
PICTORIAL GUIDE TO CLASSIFICATION OF FOOD OR DRUG PRODUCTS