ELECTRONIC LABELLING (E-LABELLING) FOR PHARMACEUTICAL PRODUCTS IN MALAYSIA
In line with digital advancement, the National Pharmaceutical Regulatory Agency (NPRA), in collaboration with relevant industry associations, has taken the initiative to implement electronic labelling (e-labelling). This initiative aims to provide consumers and healthcare professionals with detailed and up-to-date medicine information, such as the package insert (PI) and/or Consumer Medication Information Leaflet (RiMUP) that have been approved, through a QR code.
By scanning the QR code on the product packaging, users and healthcare professionals can now conveniently access additional product information quickly and accurately anytime, anywhere.
The Drug Control Authority (DCA) approved the voluntary implementation of e-labelling for pharmaceutical products in the categories of biologics, new drug products, and generic products containing scheduled poisons, at its 383rd meeting on 6th April 2023. Following this, during its 410th meeting on 3rd July 2025, the DCA further approved the expansion of e-labelling implementation to include generic products containing non-scheduled poisons or Over-The-Counter Products (OTC).
As part of efforts to strengthen this initiative, a study on the effectiveness of e-labelling implementation is currently being conducted in collaboration with Universiti Teknologi MARA (UiTM). This study aims to determine the future direction of the initiative, which remains voluntary and currently applies only to pharmaceutical products.
To ensure the study captures diverse perspectives and represents all stakeholders, feedback and input from healthcare professionals and the public are highly encouraged. Kindly access the following links to participate in the survey:
- Healthcare Professionals: https://forms.gle/CqREpopBP9gSWnEw8
- General Public: https://forms.gle/LtFdkSvRegcyvQuE9
Please refer to the infographic and watch the video provided for more information on the implementation of e-labelling.
E-LABELLING INFOGRAPHIC
Seiring arus pendigitalan, Bahagian Regulatori Farmasi Negara (NPRA) dengan kerjasama pihak persatuan industri berkaitan telah mengambil inisiatif melaksanakan electronic labelling (e-labelling), berikutan manfaatnya dalam menyampaikan maklumat ubat secara terperinci kepada pengguna dan profesional kesihatan.
Dengan hanya mengimbas kod QR pada pembungkusan produk, pengguna dan profesional kesihatan kini dapat mengakses maklumat ubat dengan lebih mudah, pantas dan terkini pada bila-bila masa, di mana jua.
Pihak Berkuasa Kawalan Dadah (PBKD), dalam Mesyuarat ke-383 pada 6 April 2023, telah bersetuju dengan pelaksanaan e-labelling secara sukarela ke atas produk farmaseutikal kategori biologik, ubat baharu dan ubat generik racun berjadual di Malaysia. Susulan pelaksanaan tersebut, PBKD dalam Mesyuarat ke-410 pada 3 Julai 2025 turut bersetuju untuk memperluaskan skop pelaksanaannya kepada ubat generik bukan racun berjadual (produk Over-the-Counter, OTC)
Sebagai langkah untuk memperkukuh pelaksanaan ini, satu kajian keberkesanan penggunaan e-labelling sedang dijalankan secara kolaboratif bersama Universiti Teknologi MARA (UiTM). Kajian ini bertujuan untuk menentukan hala tuju inisiatif berkenaan memandangkan pelaksanaannya pada masa ini masih bersifat sukarela dan hanya melibatkan produk farmaseutikal, bukan secara mandatori.
Sehubungan itu, bagi memastikan kajian ini bersifat menyeluruh serta mengambil kira pandangan semua pihak berkepentingan, maklum balas dan pandangan daripada profesional kesihatan dan orang awam amat dialu-alukan.Sila rujuk pautan berikut untuk menjawab tinjauan tersebut;
- Ahli Profesional Kesihatan:
https://forms.gle/CqREpopBP9gSWnEw8
- Orang Awam:
https://forms.gle/LtFdkSvRegcyvQuE9
Sila rujuk infografik dan tonton video yang disediakan bagi mendapatkan informasi pelaksanaan e-labelling.
E-LABELLING VIDEO
E-LABELLING INFOGRAFIK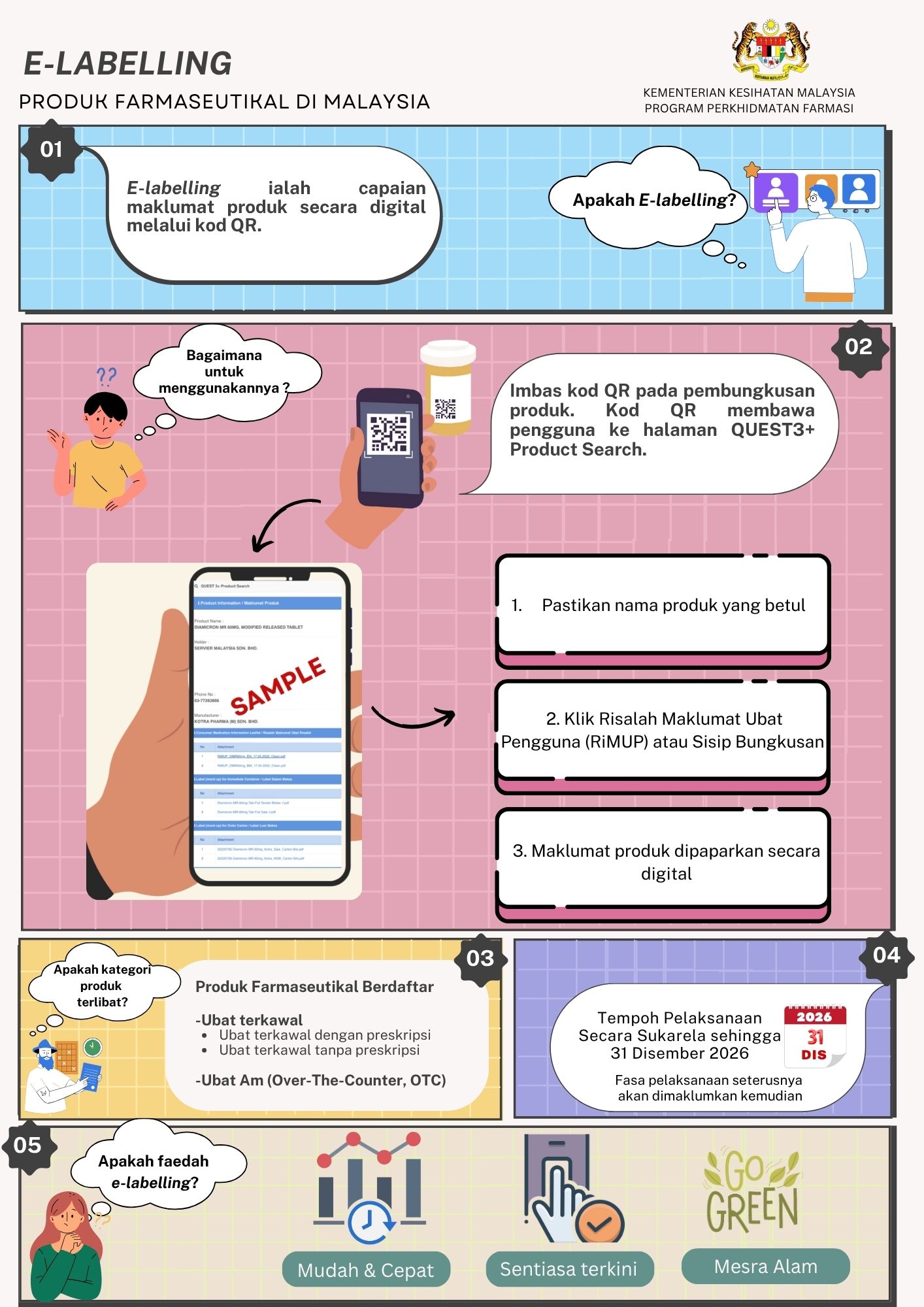
|
No |
Item |
|
|
1 |
|
|
|
2 |








