The National Pharmaceutical Regulatory Agency (NPRA) conducts Good Clinical Practice (GCP) inspections to ensure that clinical trials conducted in Malaysia comply with internationally recognised ethical and scientific standards, safeguard the rights, safety, and well-being of trial participants, while ensuring the reliability of clinical trial data.
NPRA conducts two (2) categories of GCP inspections: routine inspections and for-cause inspections. These may be carried out during an ongoing clinical trial or after its completion at investigator sites, sponsor facilities, or service providers such as CROs, clinical laboratories, or other relevant establishments. Both categories of inspections are conducted on an announced basis.
- Routine inspections are performed as part of the registration application in Malaysia. These inspections incorporate input from the Centre of Product and Cosmetic Evaluation, NPRA, particularly when issues are identified during dossier evaluation or when specific concerns are raised by the evaluators.
- For-cause inspections are initiated in response to reports, complaints or concerns of serious non-compliance, such as data integrity issues or scientific/ethical misconduct.
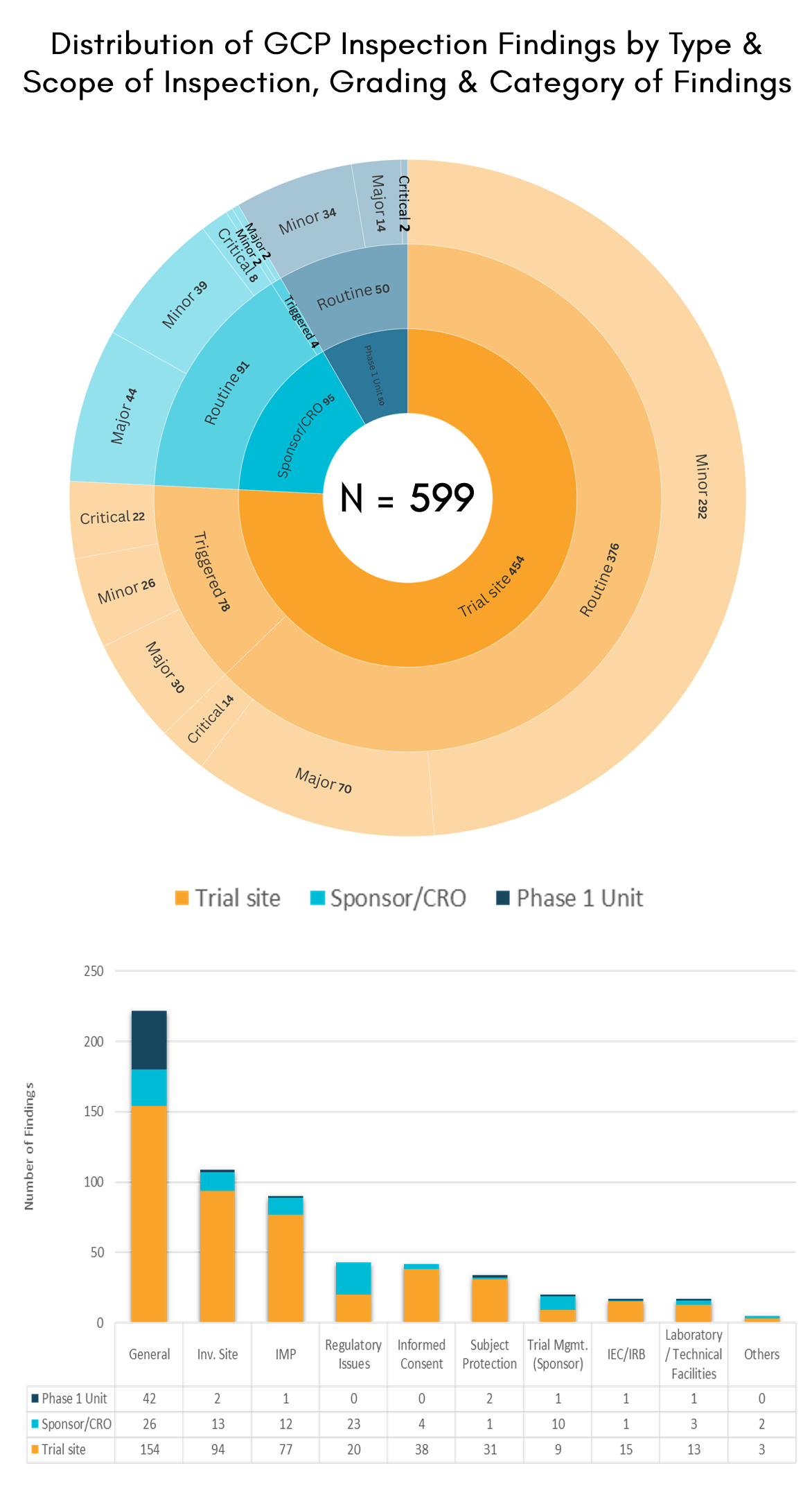
The statistics presented summarise the number of GCP inspections conducted from 2015 to 2024 and the distribution of inspection findings.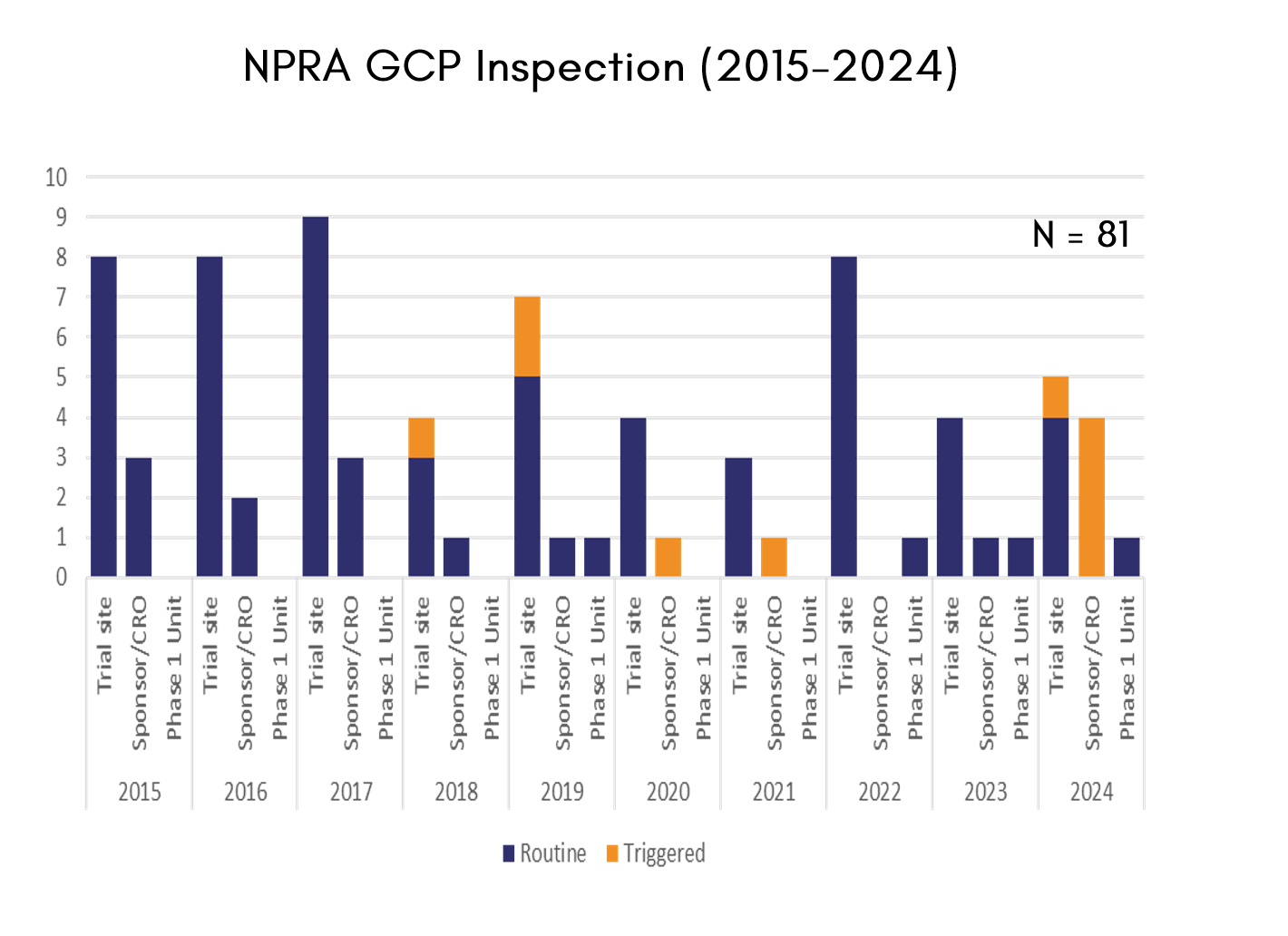
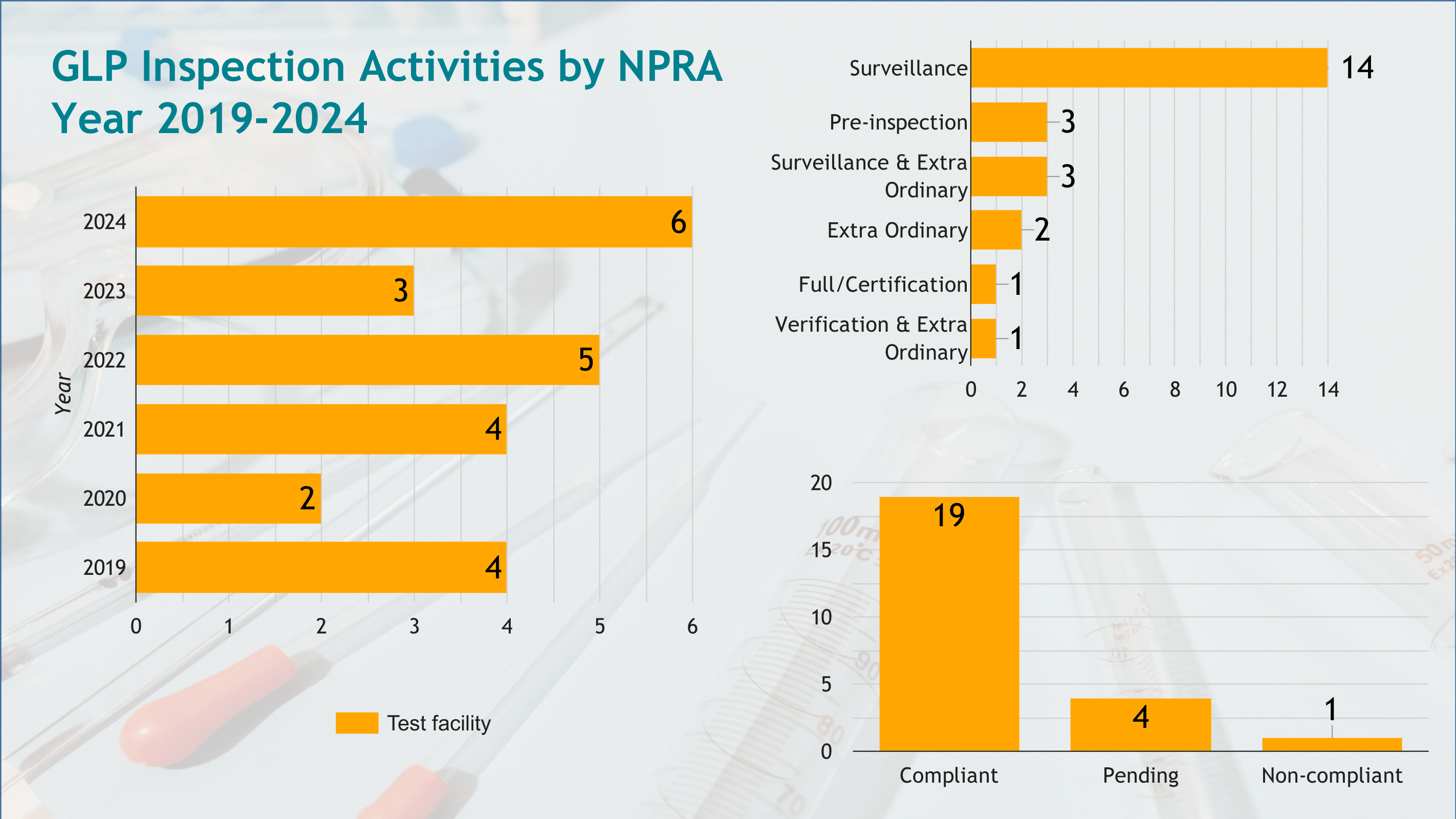
- Conducting inspections of test facilities to assess compliance with OECD GLP Principles.
- Issuing GLP compliance certificates for facilities meeting the required standards.
- Reporting inspection outcomes, including compliance status and key findings, to the OECD GLP Secretariat.
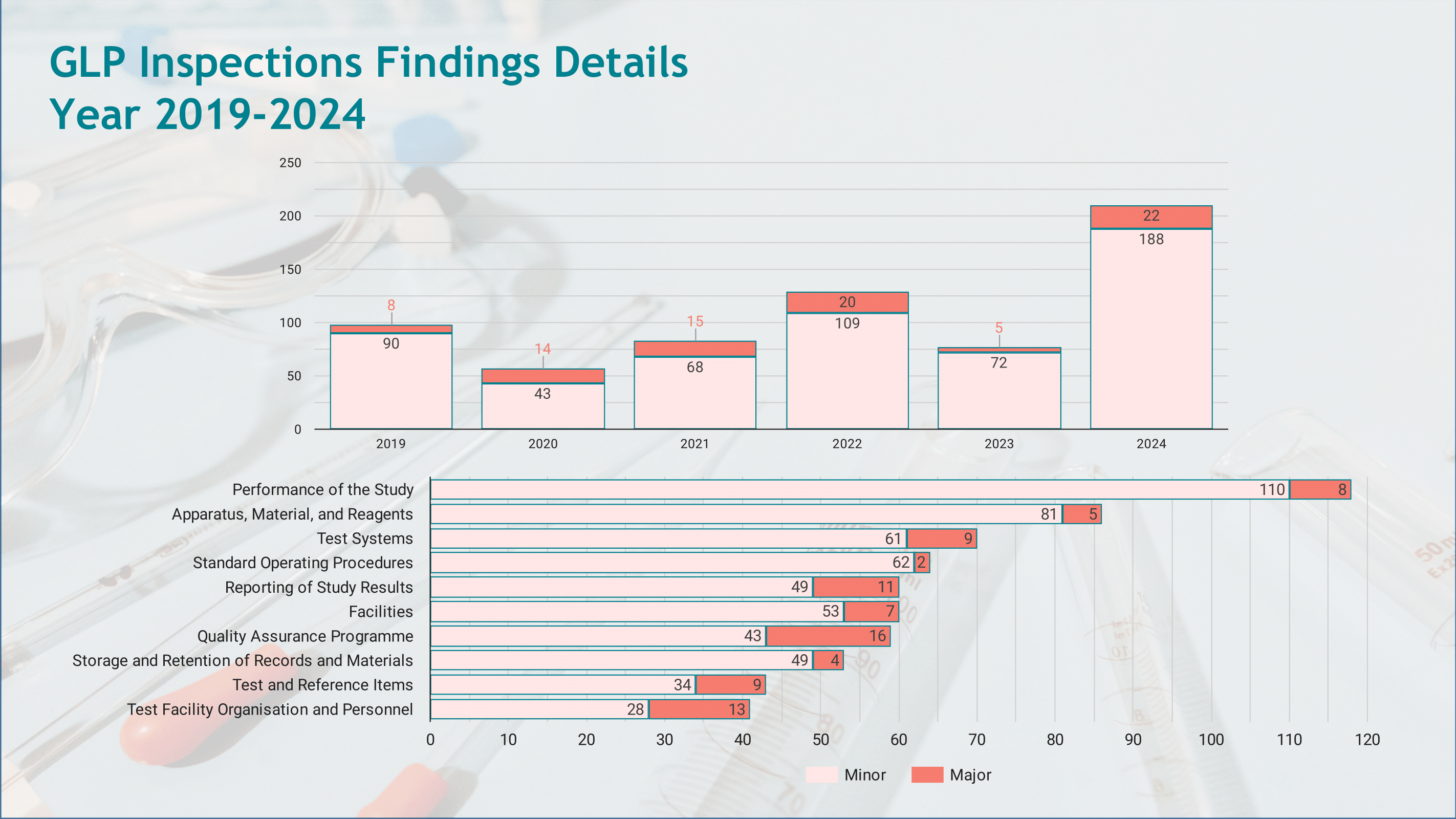
The infographic provides an overview of the inspections performed, the compliance statuses of inspected facilities, and a summary of findings, ensuring transparency and alignment with international GLP standards.