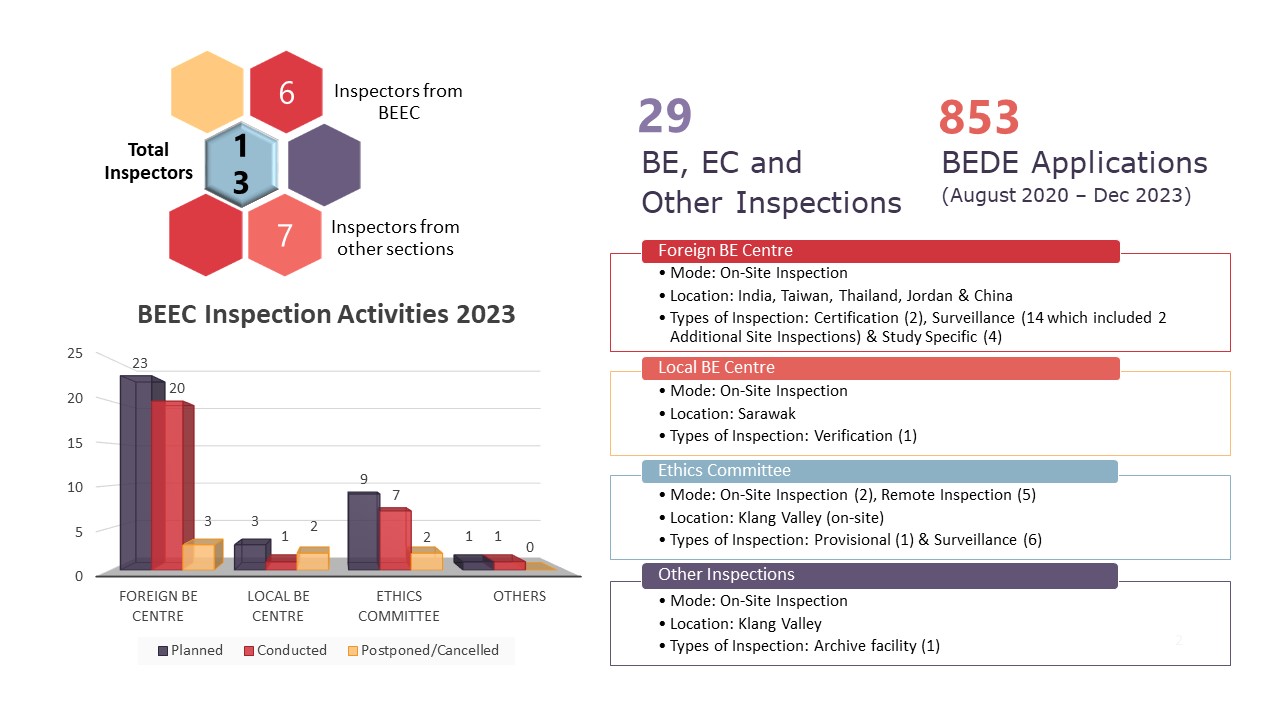
Illustration 1: Summary Infographic of BEEC Activities in 2023
The infographic above is a summary of activities that were coordinated and carried out by the Bioequivalence and Ethics Committee (BEEC) Section in 2023. 2022 saw a year in a post pandemic landscape and 2023 was the opportunity for BEEC to push the boundaries and maximise productivity of its core functionalities. In 2023, there were no changes to the manpower of BEEC. However, there were personnel movements in the Good Clinical Practice (GCP) & Good Laboratory Practice (GLP) Section and the addition of one officer from the Investigational Product & Safety Section, Centre for Product and Cosmetic Evaluation assisting in the inspections. In general, the team involved in all planned inspections are six personnel from the BEEC, five officers from the GCP&GLP Section and 2 officers from the Investigational Product & Safety Section, Centre for Product and Cosmetic Evaluation.
Extending the commitment from 2022, 2023 saw BEEC increasing its total number of inspections from 20 inspections in 2022 to 29 inspections in 2023. These inspections include 20 foreign bioequivalence (BE) centre inspections, one local BE centre inspection, seven ethics committee inspections and one inspection to a third-party archive facility classified as Other Inspections in the infographic. The only local BE centre inspection was done at Sarawak as part of a verification inspection. Of the seven EC inspections, two inspections were conducted on-site in the Klang Valley with the remaining five inspections conducted as remote inspections. Rounding up the total local inspections is the inspection to a third-party archive facility to assess how study related documents are stored on behalf of BE centres and clinical trial sites.
Foreign inspections saw an increase from seven planned inspections in 2022 to 23 planned inspections in 2023 as BEEC expands on its inspection activities. Of the 23 planned inspections, 20 were conducted which comprises of two certification inspections, 12 surveillance inspections, two additional site inspections and four study specific inspections. These inspections were conducted at BE centres located in India, Thailand, Taiwan, Jordan and China. There were three planned inspections which were not conducted in 2023. This is due to the BE centre’s decision to not continue with the listing on the NPRA BE Centre Compliance Programme (one BE centre) and the relocation of the BE centre to a new place of operations (two BE centres). The latter inspections will be postponed to a later date when the move has been completed and the BE centre resumes operations.
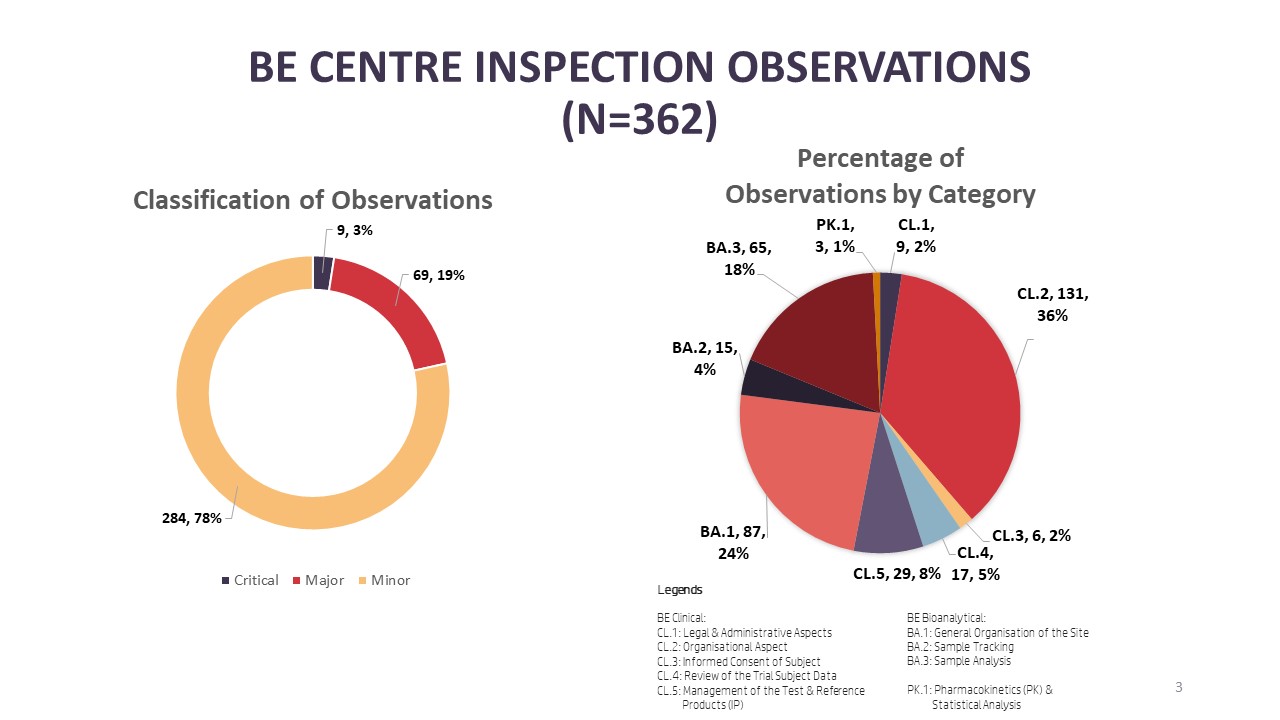
Illustration 2: Number of BE Centre Observations from Inspections Conducted in 2023
New to the infographic, BEEC is sharing information on inspection findings from inspections conducted in 2023. Starting with BE centre inspections, there were a total of 362 observations made from the total of 21 BE centre inspections in 2023. Looking into the categories that the observations were made, 36% (n=131) and 24% (n=87) were related to the organisational aspect of the clinical [CL.2] and bioanalytical facilities [BA.1] at a BE centre respectively. Completing the top three categories where observations were found are observations under study sample analysis [BA.3] with 18% (n=65). Of the 362 observations, 3% (n=9) were critical observations, 19% (n=69) were major observations and 78% (n=284) were minor observations. From the critical observations, four observations were made under the organisational aspect of the clinical facility of a BE centre [CL.2], two critical observations under the organisational aspect of a bioanalytical facility [BA.1] and three critical observations related to study sample analysis [BA.3].
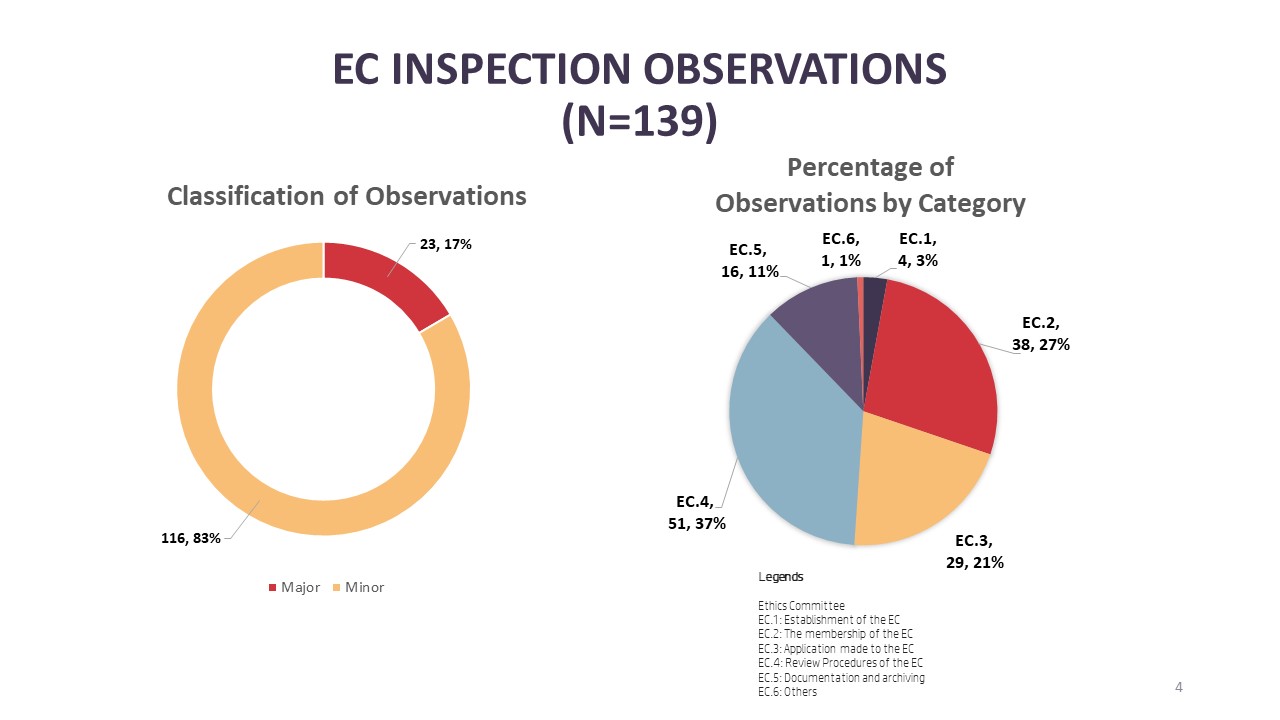
For EC inspections, there were a total of 139 observations where 17% (n=23) are major observations and 83% (n=116) minor observations. The top three categories of observations in EC inspections are under the review procedure of ECs [EC.4] with 37% (n=51) followed by membership of ECs [EC.2] with 27% (n=38) and finally procedures related to the application processing by the EC [EC.3] with 21% (n=29). As for the breakdown of major observations, the top three categories with major observations are membership of the EC [EC.2] (n=9), review procedure of the EC [EC.4] (n=7) and application processing procedures of the EC [EC.3] (n=5).
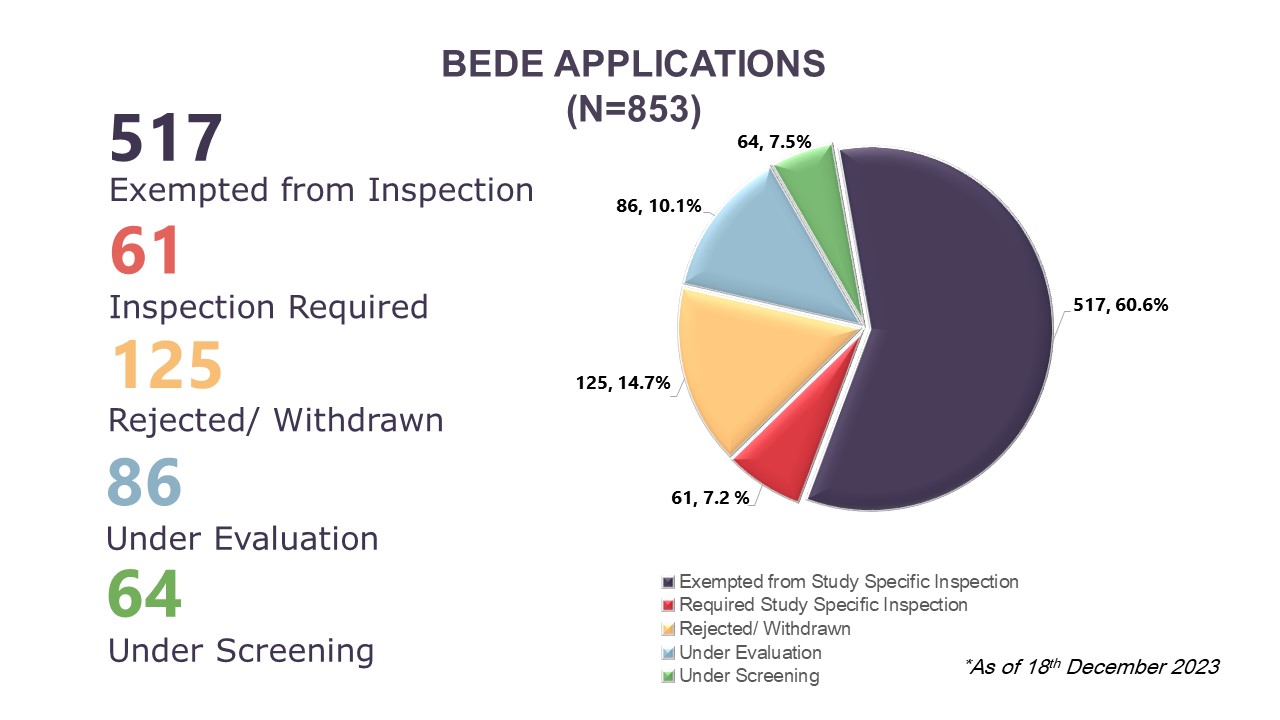
Illustration 4: Cumulative Data of BEDE Applications Received from August 2020 to 18 December 2023
On the Evaluation of the Need for BE Study Inspection (BEDE) applications, BEEC received 210 new applications, bringing the cumulative total to 853 applications received between August 2020 to 18 December 2023. Cumulatively, 67.8% (n=578) applications had received a decision where 60.6% (n=517) had received exemption for study specific inspections and 7.2% (n=61) require study specific inspections before the BE studies can be accepted to support product registration evaluation. In 2023, there were 210 BEDE applications received where 68 obtained study specific inspection exemption, four applications require inspections, and 19 applications were rejected or withdrawn by applicants. As of 18 December 2023, there are 119 applications which were received in 2023 that are still in the screening or evaluation stages. Of the 207 BEDE applications received in 2022, there are 34 applications which are still in evaluation as of 18 December 2023.
Inspections in 2023 saw several regulatory decisions and improvements carried out. In the verification inspection to a local BE centre, observations led to the upholding of the decision to suspend the BE centre from the NPRA BE Centre Compliance Programme. Observations found during the inspection to the third-party archive facility has led to the improvement of processes and the fortification of how source documents from BE studies and clinical trials stored at the archive facility will be protected.
In EC inspections, these observations led to more visibility by each of the inspected ECs to ensure that membership are free from conflicts of interest. These observations also allow future applications and review processes to be done in a more transparent and predictable way. During the inspection, ECs were also reminded on the need to have studies involving pharmaceutical products to be registered with the National Medical Research Register (NMRR) before a decision is made. Also in 2023, two ECs had decided to not continue listing under the Drug Control Authority (DCA) and the list have been updated on the NPRA website with the delisting of the ECs.
The year 2023 had given BEEC the opportunity to push the limits on the number of inspections to be conducted on top of the BEDEs received and other related functions. Going into 2024, BEEC is planning a total of 28 inspections to foreign and local BE centres, and ECs to maintain the momentum gained in 2023 to clear backlog inspections. BEEC’s commitment to process refinements will persist as we continue to balance inspections and BEDE application evaluations. The commitment to participate in stakeholder dialogues and participation in trainings for the industry will also continue in 2024.
BEEC is currently looking into streamlining the process of BEDE applications and study specific inspection applications as part of our commitment to process refinements. A public consultation with all relevant stakeholders will be done to further refine the proposal. BEEC had also been working on updating and modernising the BE centre inspection guideline and are in the final stages of internal review before releasing it for public consultation. An announcement will be issued on the NPRA and BEEC website to inform on the respective public consultations and we invite all relevant stakeholders to provide your much valued inputs. Apart from the process refinement and guideline update, BEEC will start work on updating the EC inspection guideline later this year. Additionally, BEEC will be sharing more information related to inspection findings and provide a trend analysis when more data sets are available.
At the regional level, BEEC is actively involved in the Joint Sectoral Committee (JSC) for the ASEAN Mutual Recognition Arrangement (MRA) for BE Studies. As of November 2023, the JSC MRA BE is in the process of populating its list of inspectors who will be conducting inspections on behalf of the JSC MRA BE. The JSC MRA BE had also begun accepting applications for inspections to be listed under the ASEAN MRA BE list in 2023. In line with the ASEAN MRA BE, the NPRA will be issuing a directive later in 2024 to accept all studies conducted at BE centres during valid listing on the ASEAN MRA BE list.
Prepared by: Team SBEEC, February 2024