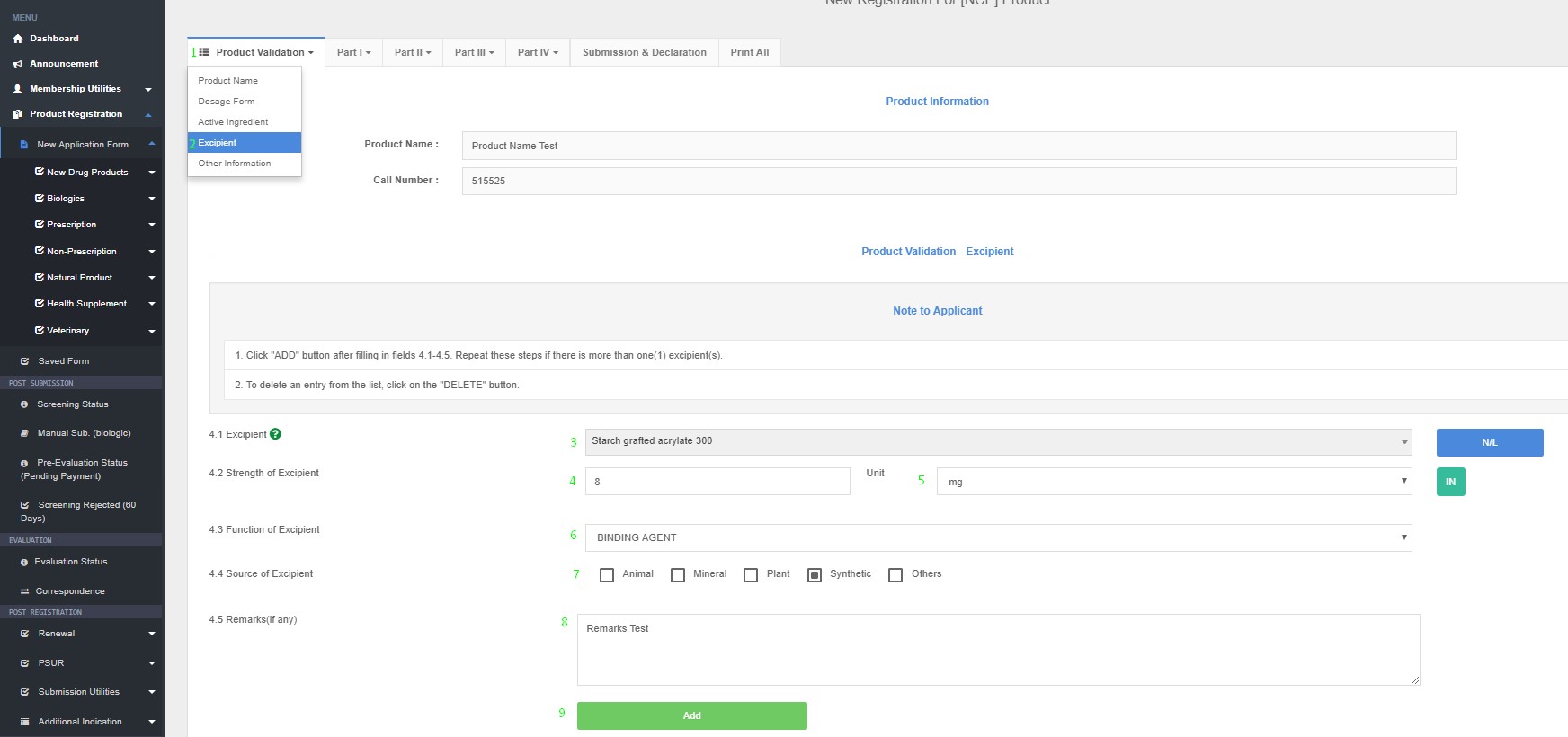
Excipient(s)
4.1 Excipient name
4.2 Strength of Excipient (Quantity unit/ dose)
4.3 Function of excipient (e.g. absorbent, diluents, bulking agent, coating agent, anti-caking agent etc.)
4.4 Source of excipient
4.5 Remarks (if any)
- For each excipient ingredient included in a formulation must have a justifiable excipient role and shall be controlled by specifications.
- New excipient will require safety and/or other additional data to support the function in the product prior to addition into the Quest 3 database.
Reference National Pharmacy Regulatory Agency (formally known as National Pharmaceutical Control Bureau):
Drug Registration Guidance Document (DRGD)-Appendix 8.2 List of Prohibited and Restricted Excipients; & Appendix 8.3 Lists of Permitted and Restricted Colouring Agents.
How to Access Excipient in QUEST3+ System ?
Product Registration >> New Application Form >> Product Validation >> Excipient