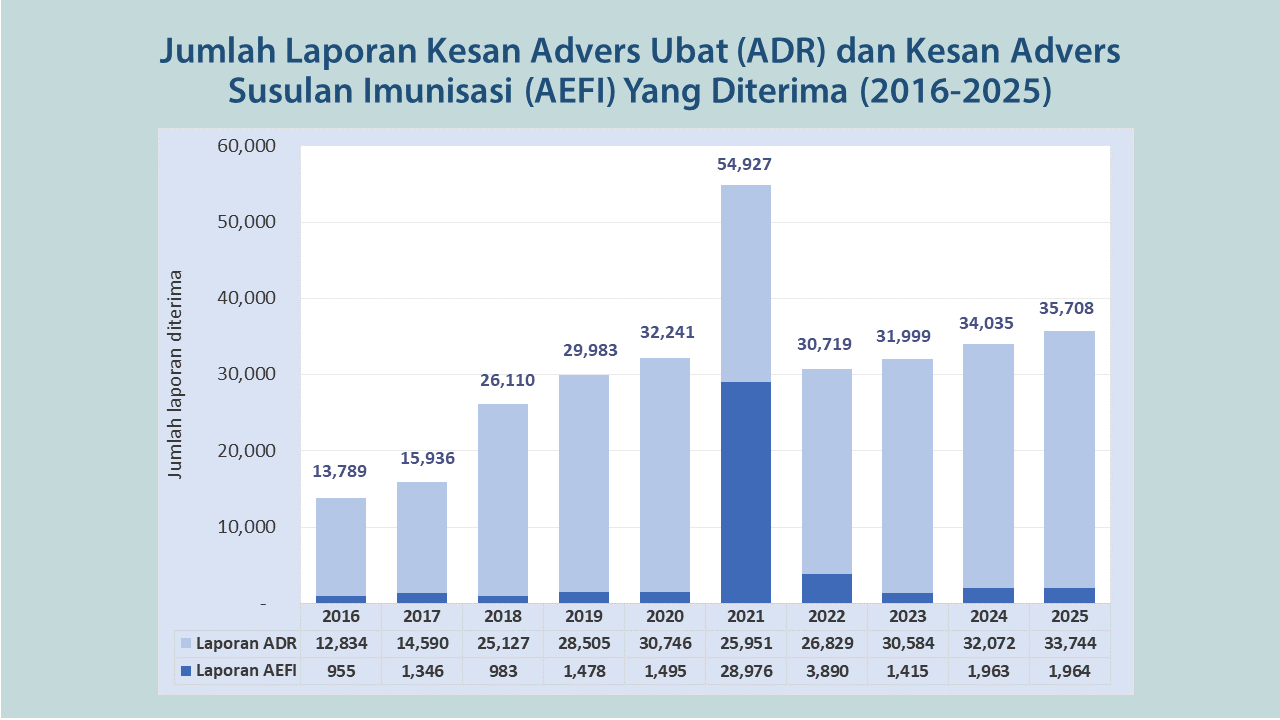
PENAFIAN: Graf di atas menunjukkan jumlah laporan yang diterima oleh pihak NPRA sebelum penilaian menyeluruh dijalankan. Kesan advers ini tidak semestinya mempunyai hubungkait dengan produk atau vaksin.

PENAFIAN: Graf di atas menunjukkan jumlah laporan yang diterima oleh pihak NPRA sebelum penilaian menyeluruh dijalankan. Kesan advers ini tidak semestinya mempunyai hubungkait dengan produk atau vaksin.
National Pharmaceutical Regulatory Agency (NPRA)
Lot 36, Jalan Universiti (Jalan Prof Diraja Ungku Aziz), 46200 Petaling Jaya, Selangor, Malaysia.
The Government of Malaysia and the National Pharmaceutical Regulatory Agency are not responsible for any loss or damage caused by the usage of any information obtained from this website.