- Listed active ingredients can be checked through http://npra.moh.gov.my/ of product search. Ingredients not listed will require safety and/or efficacy data evaluation prior to addition to this list.
- Substances that are included in the formulation as active ingredients must make a contribution to the proposed indications for the product.
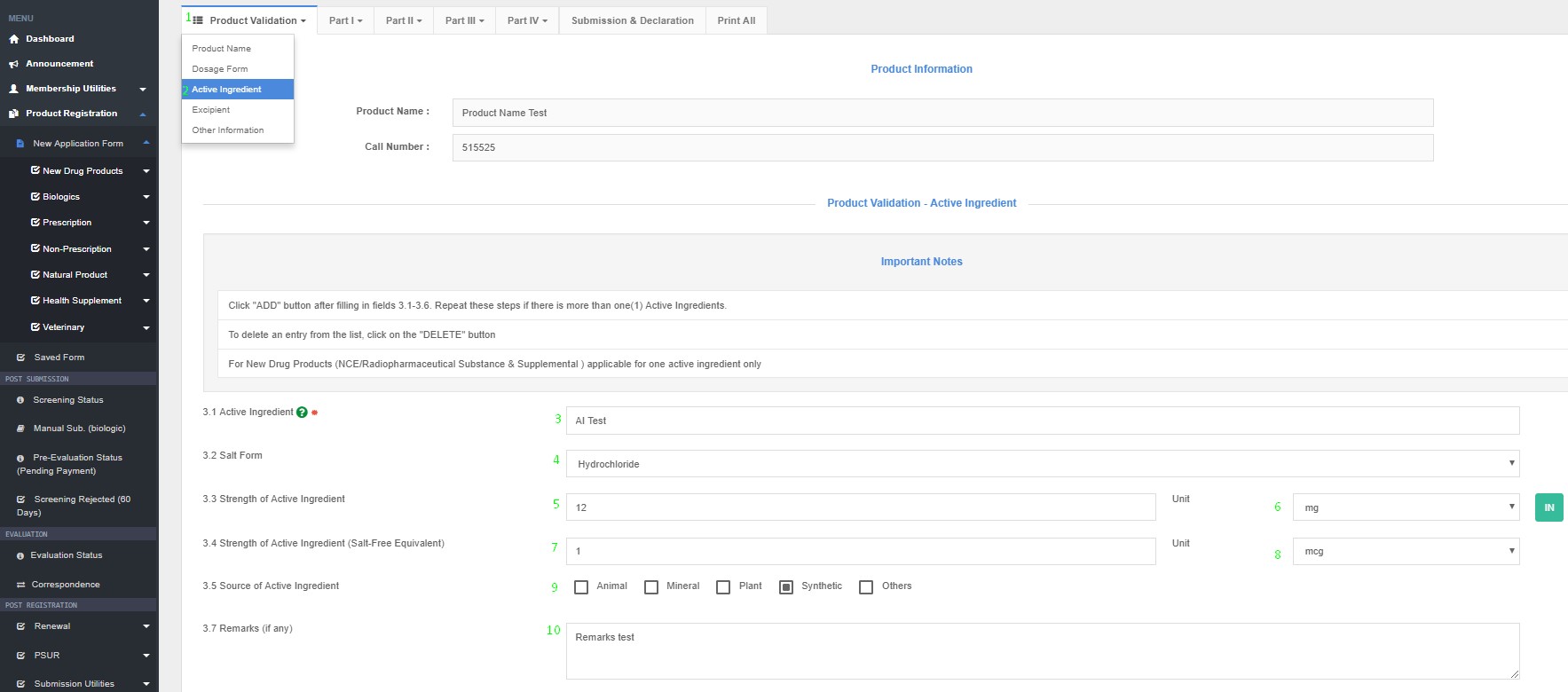
- Please specify the source such as animal, plant, synthetic or others.
- Please check whether the product contains active ingredients listed in the PROTECTED/ ENDANGERED WILDLIFE OR BOTANICAL SPECIES as in the DRGD.The applicant shall contact the appointed department as listed in DRGD to obtain the necessary permit / license.
Strength of active ingredient :
- To enter the content of active ingredients (numerical) and then select the weights and measures from the given list.
- The content of ingredients shall be expressed as appropriate in the following manner: a) quantity per dose unit (e.g. for unit dose formulations - tablet, capsule, lozenge, etc.) b) percentage composition - %w/w, %w/v, %v/v, etc. c) weight per ml. (e.g. for solutions, suspension etc.)
- Quantity (percentage or amount) per measured dose (e.g. oral liquids, drops, etc.)
- Metric weights and measures shall be used.
- Effective from 1 December 2007, premixed ingredient(s) shall not be used in a traditional product formulation, as directed in circular Bil (71) dlm BPFK/02/5/1.3.
- Please ensure that only active substances that are permitted and no substance are prohibited. Please refer to Appendix 5 under 2.1.3 'Prohibited / Banned ingredients' in DRGD.
- Please ensure the Aristolochic Acid test from governmental doping center is submitted in F12 for products containing active ingredients listed in List A - Botanicals Known or Suspected to contain Aristolochic Acid and List B - Botanicals which may be adulterated with Aristolochic Acid.
- Please take note this specific ingredients not allowed to be registered under Traditional Medicine:
- a) Crinis Carbonisatus (Carbonised human hair) b) Human Placenta
- For new active ingredients or new combination products, the following information shall be required:
a) Product containing new single ingredient:
i) Extract form
- Information on the taxonomy of the ingredient;
- Techniques and methods in preparing/ processing the extract and subsequently the product;
- Information on the use and safety of the ingredient and the product quality standard. ii) Powder/ Granules
- Information on the taxonomy of the ingredient;
- Techniques and methods in preparing/ processing the extract and subsequently the product;
- Information on the use and safety of the ingredient and the product.
b)Product containing multiple ingredients (contains ingredients which are known to be used traditionally):
- The source of the product formulation; e.g. Chinese Pharmacopoeia
- Proof or evidence of the use, traditionally.
c) Product containing multiple ingredients (contains ingredients which are not known to be used traditionally):
- Information on the use and safety of every new ingredient;
- Safety data on the new formulation;
- Regulatory status in other countries.
Reference National Pharmacy Regulatory Agency (formally known as National Pharmaceutical Control Bureau):
Drug Registration Guidance Document (DRGD) - Appendix 8.1 List of Prohibited and Restricted Active Ingredients and Combinations.
How to Access Active Ingredient in QUEST3+ System ?
Product Registration >> New Application Form >> Product Validation >> Active Ingredients